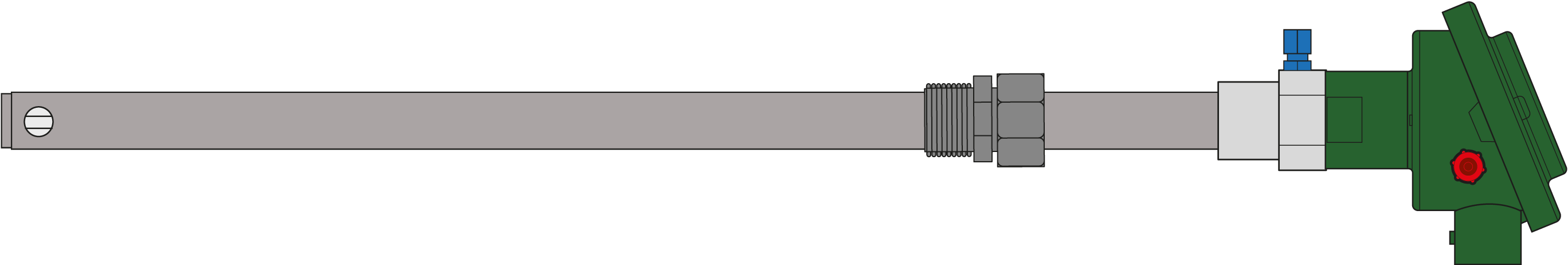Products comparison
Products comparison
The principle of operation of an oxygen sensor
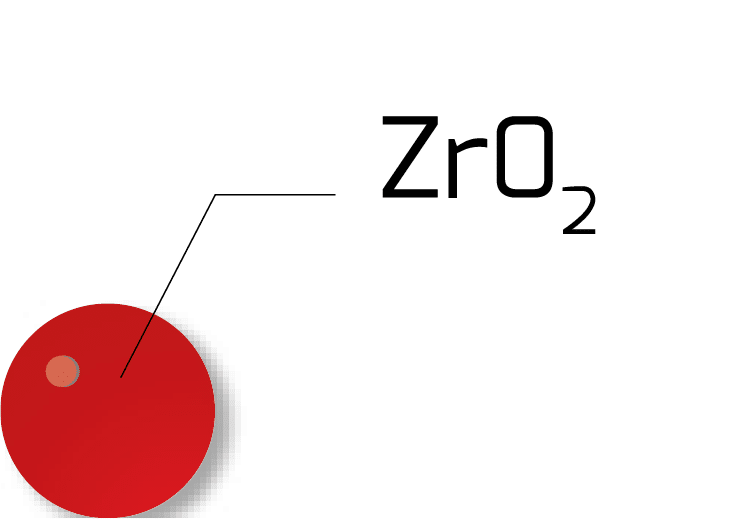
The operating principle of an oxygen probe is the comparison of two partial pressures of oxygen in two separate gaseous media. The zirconium oxide (ZrO2) in the measuring cell has lattice defects – in other words, some of the sites that could be occupied by oxygen ions are missing. In order to compare these partial pressures, the oxygen probe must be supplied with a gas whose oxygen content is known (ambient air: %O2 = 20.9%), this is the so-called baseline air.
The property of this ceramic allows the displacement of these oxygen ions at a temperature above 600 °C. The measuring element then becomes conductive. The voltage thus generated expresses a ratio between the relative difference in oxygen concentrations and the temperature of the process atmosphere.
Once the voltage value (which varies between 0 and 1200 mV) has been measured, the oxygen concentration can be easily calculated using the Nernst equation to obtain %O2 or by knowing the CO content (either by analysis or by theoretical calculation). The carbon potential (%C) of the heat treatment atmosphere can then be determined.
Econox uses 3 different types of ZrO2 cells for its product line :
| ZrO2 Ball |
Complete ZrO2 Cell | Mixed ZrO2 Cell | |
 |
 |
||
| Dimension | Ø 14mm | 8 x 6mm | 8 x 6mm |
| Max Length (mm) | – | 1 000mm | 1 400 mm |
| Thermal shock resistance | Extreme | Low | Normal |
| Econox products |
|
|
|